Volunteer citizens taking part in clinical trials of Chinese vaccine (photos)
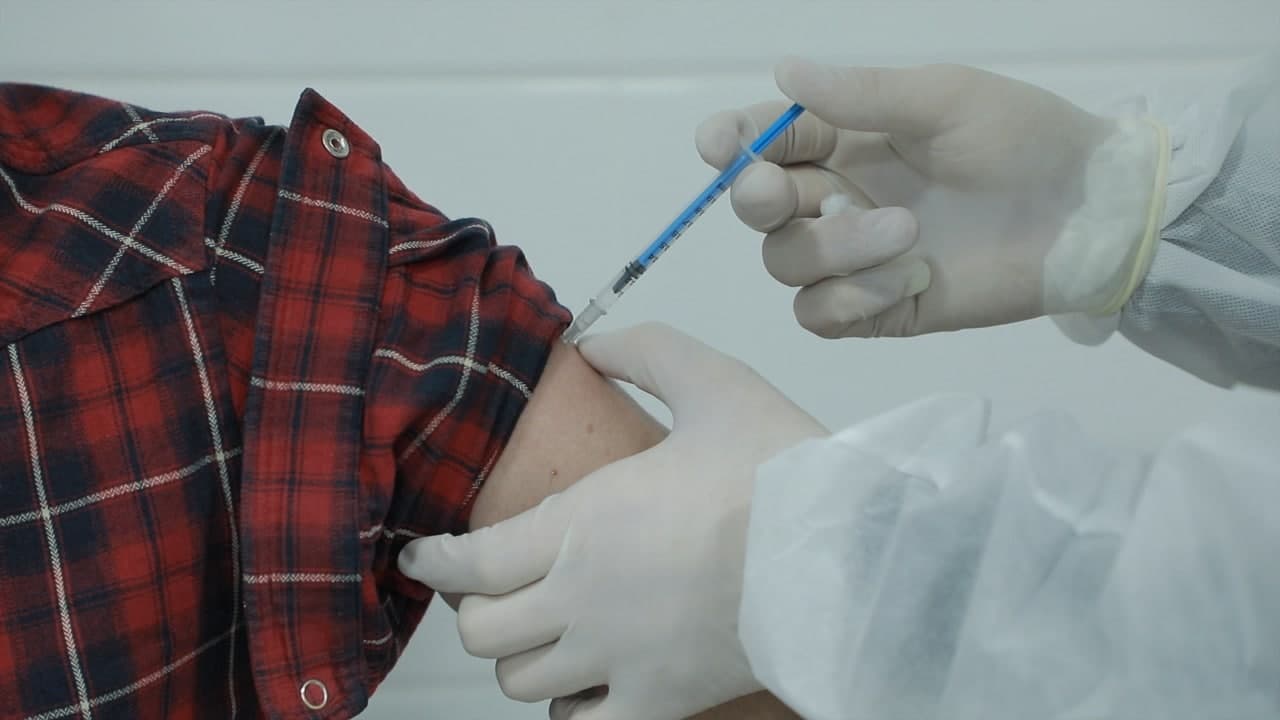
Five thousand volunteers over the age of 18, who do not have chronic illnesses, are being vaccinated in Uzbekistan. They have received the first dose of the vaccine and are scheduled to receive a total of 3 doses.
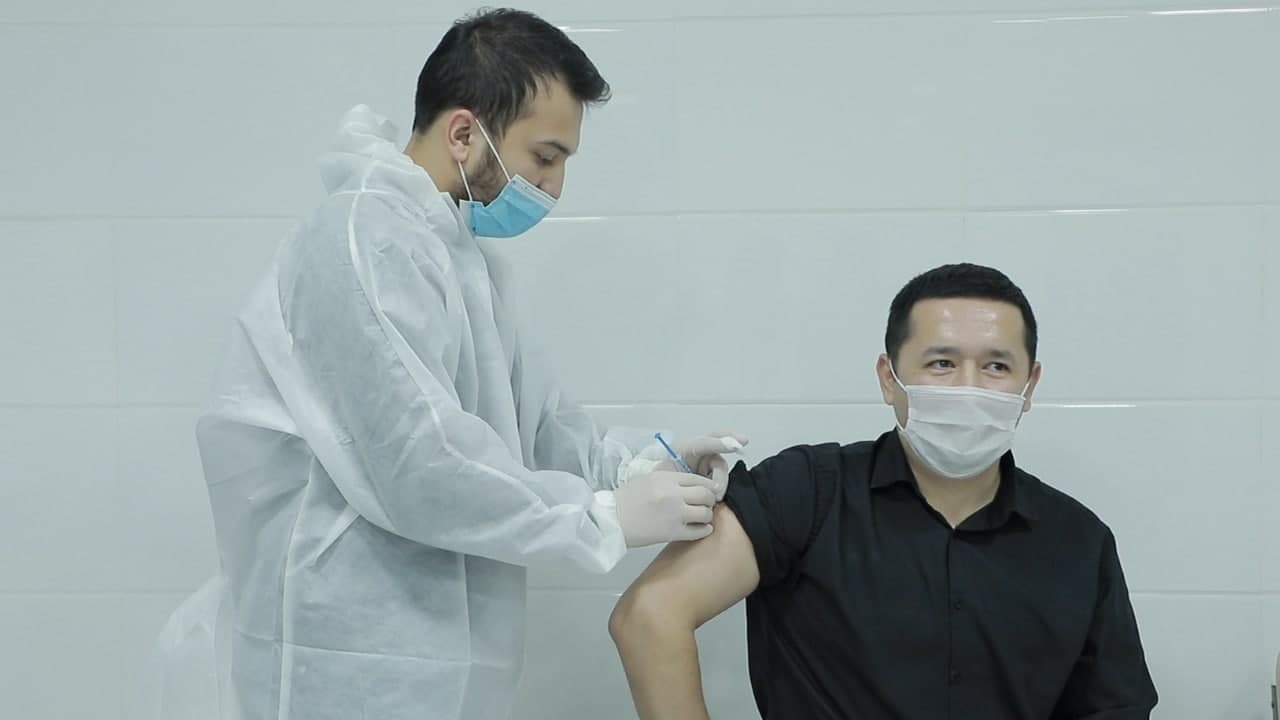
Photo: Ministry of Innovative Development of Uzbekistan
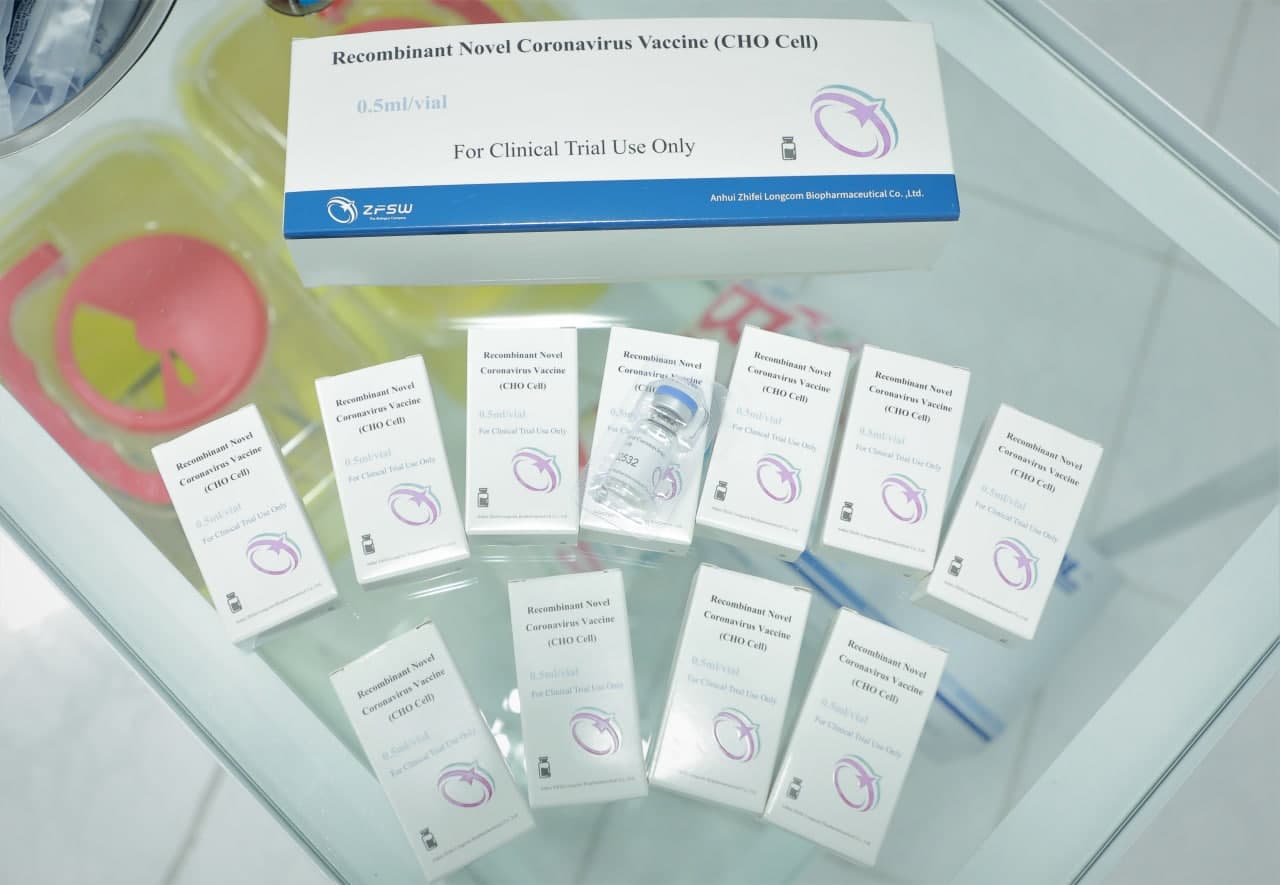
The Center for Advanced Technologies under the Ministry of Innovative Development of Uzbekistan and the Research Institute of Virology of the Health Ministry are conducting phase III clinical trials of the recombinant vaccine ZF2001 produced by the Chinese company Anhui Zhifei Longcom Biopharmaceutical.
According to the Ministry of Innovative Development, it is planned to involve 5,000 volunteers over the age of 18 who do not have chronic diseases. Volunteers will be vaccinated with a total of three doses of the vaccine during the trial period.
A trial vaccine is a type of vaccine developed as a result of research and is intended for use in clinical trials. It will not be sold until approved by the relevant authorities. The vaccine was prepared by purifying the recombinant form of a new coronavirus receptor-binding protein (NCP-RBD) produced in CHO cells by adding aluminum hydroxide.
It is noted that participation or non-participation in this study is entirely voluntary. Volunteers are not forced to continue the test during the probationary period. Candidates who decide to take this test are asked to sign a letter of consent and keep a copy of it. Before deciding to participate in the test, it should be confirmed that there are no symptoms associated with COVID-19 and that there is no doubt that you are infected with COVID-19. The candidate must also confirm that he or she has not participated in the COVID-19 vaccine test.
“The medical history, health indicators, data collected after receiving the vaccine and other similar information of the candidate, who decided to participate in the study, will be recorded. At the same time, candidates will be instructed on how to avoid a new coronavirus infection.
The test will last 14 months,” the report said.


Related News

14:50 / 30.05.2025
Health official: No grounds for alarm over COVID-19 mutations in Uzbekistan

15:08 / 25.04.2025
BioVitrum plans to establish a scientific and industrial complex in Tashkent

21:10 / 12.02.2025
"Certificate of meningitis vaccination for Umrah and Hajj pilgrims not canceled" – Sanitary and Epidemiological Committee
14:42 / 06.01.2025




