Gov’t to start labeling of medicines and medical devices from September 1
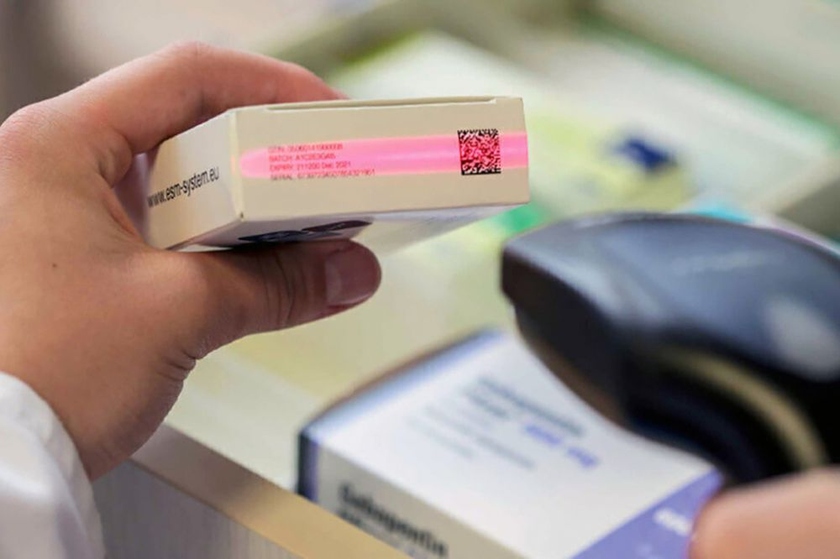
Photo: zdrav.expert
On April 2, the Cabinet of Ministers adopted a resolution No. 149 “On introducing a system of mandatory digital marking of medicines and medical devices”, Norma reported.
The document envisages the gradual introduction of a mandatory digital labeling system by means of identification in 2022-2025, taking into account the grouping of drugs (excluding substances) and medical devices.
Terms of commencement of obligatory digital marking and types of products are defined as follows:
• the 1st group – medicines produced in secondary (outer) packaging (except for orphan medicines) – from September 1, 2022;
• the 2nd group – medicines and medicinal angro-products (except for orphan medicines) produced in primary (internal) packaging – from November 1, 2022;
• the 3rd group – orphan drugs (for the treatment of rare diseases) – from March 1, 2023;
• the 4th group – specially registered imported medical devices – from February 1, 2025.
The Cabinet of Ministers approved the following:
• list of identification codes of medicines and medical devices with mandatory digital marking;
• schedule of gradual introduction of mandatory digital labeling system by local manufacturers of medicines and medical devices;
• schedule of gradual introduction of mandatory digital labeling system by foreign manufacturers of medicines and medical devices;
• schedule of gradual transition of wholesale and retail organizations to the system of mandatory digital labeling of medicines and medical devices;
• schedule of gradual transition of medical institutions in the field of health care to the system of mandatory digital labeling of medicines and medical devices;
• regulations on the procedure for digital marking of medicines and medical devices by means of identification.
The document was published in the National Database of Legislation and came into force on April 2.
Related News

10:07 / 06.02.2026
Pharmacies in Uzbekistan earned billions through inflated drug prices in 2025

21:24 / 21.01.2026
US deports 44 Uzbek nationals on charter flight to Tashkent

21:07 / 21.01.2026
Uzbekistan expands pharmaceutical reforms with price cuts and digital prescriptions

16:40 / 13.01.2026



